Software As A Medical Device Labeling Requirements . as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. medical device qms requirements when the patient safety perspective is included. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. this document provides guidance on defining intended purpose for software as a medical device (samd). this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. It is intended to assist samd manufacturers in meeting. This document highlights elements of good.
from mavink.com
This document highlights elements of good. medical device qms requirements when the patient safety perspective is included. It is intended to assist samd manufacturers in meeting. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. this document provides guidance on defining intended purpose for software as a medical device (samd). as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software.
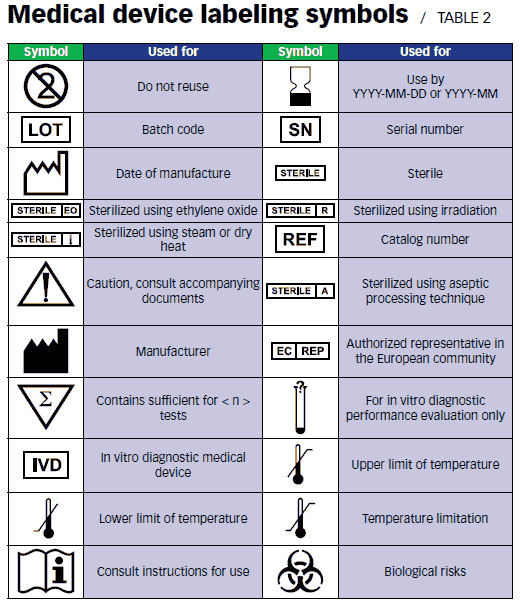
Medical Device Labeling Symbols
Software As A Medical Device Labeling Requirements as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. This document highlights elements of good. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. this document provides guidance on defining intended purpose for software as a medical device (samd). medical device qms requirements when the patient safety perspective is included. It is intended to assist samd manufacturers in meeting. this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier.
From www.teklynx.com
Why TEKLYNX is the Best Labeling Software for Medical Devices Software As A Medical Device Labeling Requirements this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. This document highlights elements of good. this document provides guidance on defining intended purpose for software as a medical device (samd). the term software as a medical device is defined by the international medical device regulators forum (imdrf). Software As A Medical Device Labeling Requirements.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Software As A Medical Device Labeling Requirements this document provides guidance on defining intended purpose for software as a medical device (samd). It is intended to assist samd manufacturers in meeting. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. as a manufacturer of software as a medical device, you must ensure that you. Software As A Medical Device Labeling Requirements.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Software As A Medical Device Labeling Requirements medical device qms requirements when the patient safety perspective is included. this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. this post provides information on the medical. Software As A Medical Device Labeling Requirements.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Software As A Medical Device Labeling Requirements this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. this document provides guidance on defining intended purpose for software as a medical device (samd). This document highlights elements. Software As A Medical Device Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Software As A Medical Device Labeling Requirements this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements. Software As A Medical Device Labeling Requirements.
From www.slideshare.net
Symbols Commonly Used in Medical Device Packaging and Labeling Software As A Medical Device Labeling Requirements This document highlights elements of good. medical device qms requirements when the patient safety perspective is included. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. this document provides guidance on defining intended purpose for software as a medical device (samd). the term software as a. Software As A Medical Device Labeling Requirements.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Software As A Medical Device Labeling Requirements this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. medical device qms requirements when the patient safety perspective is included. this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. as a manufacturer of software as a. Software As A Medical Device Labeling Requirements.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Software As A Medical Device Labeling Requirements this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. It is intended to assist samd manufacturers in meeting. as a manufacturer of software as a medical device, you. Software As A Medical Device Labeling Requirements.
From andamanmed.com
Medical device labeling requirements in the Philippines Software As A Medical Device Labeling Requirements this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. medical device qms requirements when the patient safety perspective is included. This document highlights elements of good. It is intended to assist samd manufacturers in meeting. as a manufacturer of software as a medical device, you must ensure. Software As A Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Software As A Medical Device Labeling Requirements as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. medical device qms requirements when the patient safety perspective is included. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. this post provides information on the. Software As A Medical Device Labeling Requirements.
From ceaokqsc.blob.core.windows.net
Medical Devices Labels at John Hernandez blog Software As A Medical Device Labeling Requirements This document highlights elements of good. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. It is intended to assist samd manufacturers in meeting. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. medical device qms requirements. Software As A Medical Device Labeling Requirements.
From clin-r.com
Labels for Medical Devices Clin R Software As A Medical Device Labeling Requirements this document provides guidance on defining intended purpose for software as a medical device (samd). medical device qms requirements when the patient safety perspective is included. the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. It is intended to assist samd manufacturers in meeting. This document highlights. Software As A Medical Device Labeling Requirements.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Software As A Medical Device Labeling Requirements as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. this guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. this document provides guidance on defining intended purpose for software as a medical device (samd). this post. Software As A Medical Device Labeling Requirements.
From www.afpharmaservice.com
Medical Device Labelling Requirements Software As A Medical Device Labeling Requirements the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. This document highlights elements of good. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. It is intended to assist samd manufacturers in meeting. this guidance document describes. Software As A Medical Device Labeling Requirements.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Software As A Medical Device Labeling Requirements medical device qms requirements when the patient safety perspective is included. This document highlights elements of good. It is intended to assist samd manufacturers in meeting. as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. the term software as a medical device is defined by the. Software As A Medical Device Labeling Requirements.
From satoasiapacific.com
SATO Medical Device Barcode Labelling Solution SATO AutoID Malaysia Software As A Medical Device Labeling Requirements medical device qms requirements when the patient safety perspective is included. as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. It is intended to assist samd manufacturers in meeting. this post provides information on the medical device labelling requirements and user manual requirements under the eu. Software As A Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Software As A Medical Device Labeling Requirements the term software as a medical device is defined by the international medical device regulators forum (imdrf) as software. this document provides guidance on defining intended purpose for software as a medical device (samd). as a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before. This document highlights. Software As A Medical Device Labeling Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Software As A Medical Device Labeling Requirements It is intended to assist samd manufacturers in meeting. this post provides information on the medical device labelling requirements and user manual requirements under the eu mdr 2017/745. this document provides guidance on defining intended purpose for software as a medical device (samd). this guidance document describes the general labelling principles for medical devices and ivd medical. Software As A Medical Device Labeling Requirements.